Chromatin and genome stability
One of the challenging questions has been understanding how DNA repair machinery operates in eukaryotic cells, where DNA is embedded in chromatin. Our previous work provided some important insights into this process. Our studies led to the identification of a SNF2 ATPase ALC1, a novel DNA repair factor with chromatin remodelling function (Ahel et al, Science. 2009). ALC1 is recruited to sites of DNA damage by its interaction with a specific post-translational modification: poly(ADP-ribose). Poly(ADP-ribose) is rapidly synthesised at sites of DNA damage by poly(ADP-ribose) polymerase 1 (PARP1), and our data show that it acts as an essential signal for the recruitment of a range of DNA damage response factors, including ALC1 (Ahel et al, Nature. 2008; Ahel et al, Science. 2009). Recruitment of ALC1 to sites of DNA damage is strictly dependent on active poly(ADP-ribose) synthesis and is abrogated upon chemical inhibition of poly(ADP-ribosyl)ation by PARP inhibitors.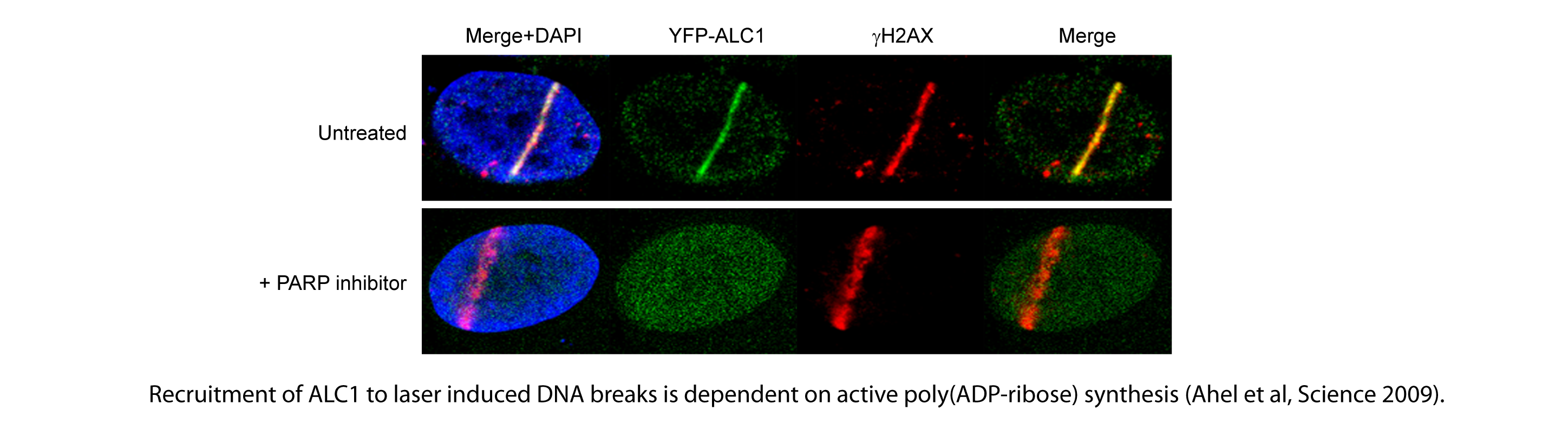
ALC1 is a modular protein, and its DNA repair and chromatin remodelling functions are supported by two distinct domains. Recruitment of ALC1 to sites of DNA damage is mediated by its C-terminal Macro domain, which acts a poly(ADP-ribose)-binding module. In contrast, chromatin remodelling activity is supported by the N-terminal SNF2 ATPase core, related to the analogous domains found in the SNF2 family of ATP-dependent chromatin remodelling enzymes (such as SNF2, ISWI and CHD1). Therefore, a unique combination of functional domains allows ALC1 to target its chromatin remodelling activity to precise chromatin locations and support DNA repair.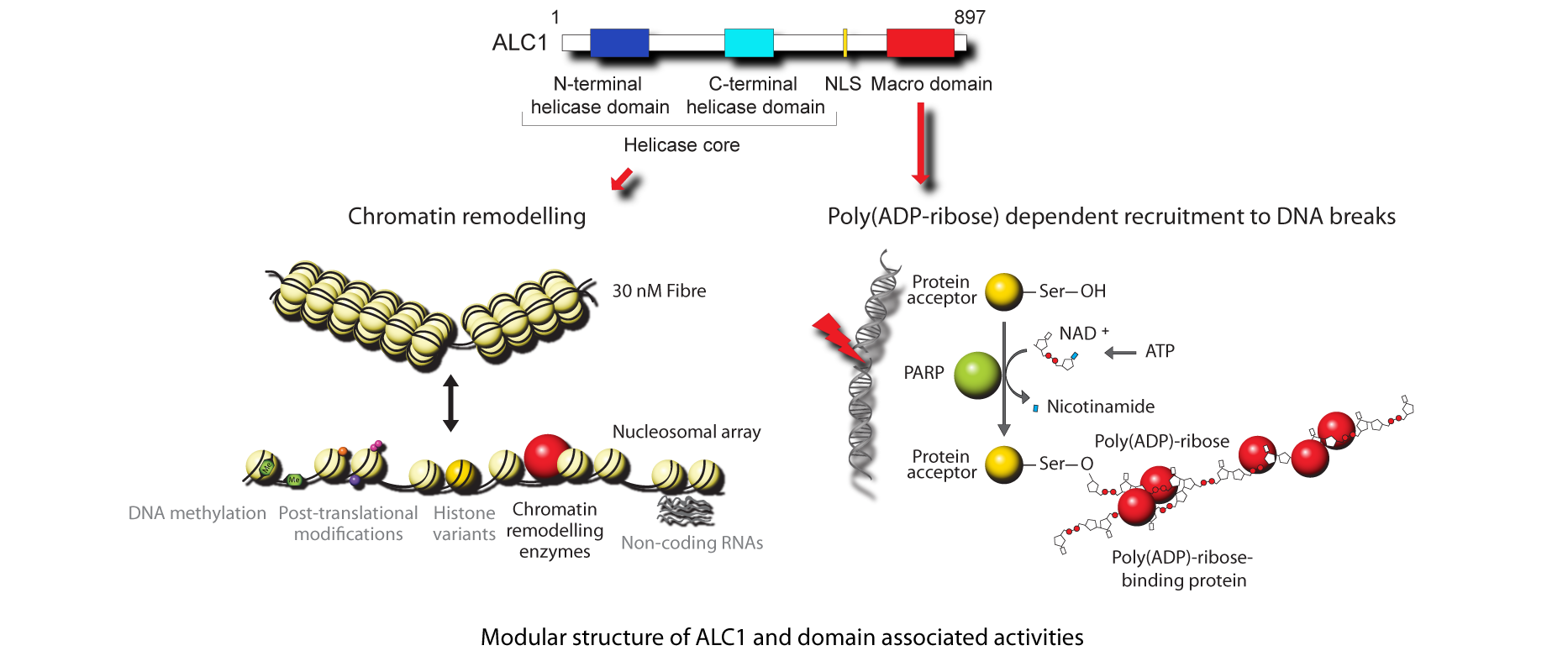
Importantly, ALC1 is frequently amplified and overexpressed in human hepatocellular carcinoma (HCC), which has important clinical implications. HCC is among the most lethal of human malignancies and the third-leading cause of cancer-related deaths worldwide. Despite this, HCC is understudied compared to other major lethal cancers and the molecular mechanisms responsible for its aggressive nature are poorly understood. Our aim is to uncover the functional link between ALC1 activity and tumourigenesis using a combination of in vivo and ex vivo approaches.